New Product Development Intern (R&D) @ CooperSurgical, Inc

Non-Disclosure: Only shareable highlights included; proprietary details remain confidential.
CooperSurgical is a leading medtech company with a strong pipeline of fertility and women’s health products. During my internship, I contributed to FDA Class II/III device programs across product design, process development, and manufacturing readiness collaborating with engineering, industrial design, and clinical teams.
Key Contributions
- Initiated and prototyped Auto Integra, a first-of-its-kind FBW ICSI micromanipulator add-on that introduced submicron precision, segmentation, manual override, and seamless IVF workflow integration. The system was demoed at an internal summit, secured continued investment, and advanced toward preclinical trials validated on Volvox and mouse oocytes.
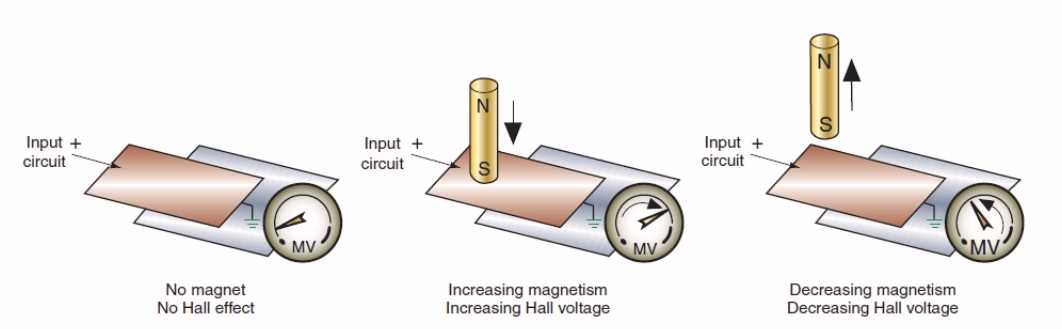
- Designed and validated subsystems including incubator lids with integrated neodymium magnets and hall sensor proximity systems, thermal and airflow module redesigns, and accelerated aging rigs to ensure mechanisms met rigorous FDA quality requirements.
- Conducted bench testing and usability studies, performed bubble tests for Class III IUDs, and supported packaging verification for Class III fertility devices.
- Built prototypes for new products and internal tools, including rapid prototyping and material selection for incubator subsystems.
- Collaborated with cross-functional teams to accelerate product development, scale internal tools, and support readiness for upcoming product launches.